How to make a good Iron saturated red in cone 6 oxidation. I've done it.
Here I will lay out a full breakdown of my version of ancient copper as well as in depth notes to my process. Before I start this blog I want to say a few things. Firstly this is not THE ancient copper recipe, I dit not sneak into Amazon and take it from this. This is my own version of the glaze that I figured out with the help of a friend and the experience I have behind me. Secondly I want to give a shout out to Reinout Brouwers who helped in the for of letting me bug him with questions about glaze chemistry while I rolled over some things. He probably saved me months of testing. OK, let’s get it done.
Amaco’s Ancient Copper glaze went off market ( I'm assuming because of the Gerstly Borate shortage as they happened around the same time) some time ago. I told myself I would recreate this glaze as it seemed to just be a iron crystal glaze. I knew how to create a crystal glaze so I figured it wouldn't be that hard. I startd at the bottom left of the stull chart and dumped zinc into one of my crystal base glazes. Of course it didn't work.
I spent about 2 months here. What I didn't understand is zinc (the main crystal former) and iron don't get along to well in the amount needed to create crystals. Even when I got it to work my goal was to make the glaze fluid/run enough to show or have crystals be present, This led to a line test I called “test fluid” or T.F when written on the tests. This was a test with frit 3110 (a lot of crystal glaze bases have this frit), zinc, and titanium to see if it did anything. Here is a sample.
Note: when you see an upright test tile I almost always double dip them from the half way up point. So from the halfway down point it’s one dip while half way up is a second dip/layer.
I did many more test than this, usually between 5-10-15% zinc and 2-3-4% titanium. Many of these tests didn't even turn out red let alone crystals so this line of tests was dead. I then started to think that it was not a crystal glaze but instead a Matt glaze. so I jumped to the Matt part of the stull chart. Usually having more alumina than silica. At this point I did not know what made a good red, chemically that is. I did how every have about 3 glazes in my name that were very red.
Here is where it gets frustrating. A friend of mine pointed me to an article that claims to know how to make the perfect red glaze in oxidation. I figured, If I can make a good red glaze then I can just add zinc to it and BOOM Ill have a crystal red! That was incorrect. So I spent about three months testing for matt iron reds after reading this article. https://cone6pots.ning.com/m/discussion?id=2103784%3ATopic%3A63380 . A quick summary of this article states that “ The best iron reds in oxidation firing are obtained with about 0.10 molar CaO, 0.03-0.06 MgO, 0.01-0.02 P2O5 and 0.08-0.12 FeO.”
None of the reds I have made have ever fit this chemistry. It essentially says “for every 0.1 amount of calcium you can have / should balance the magnesium, iron, and phosphorus accordingly. 0.03-0.06 magnesium, 0.01-0.02 phosphorus, ect. Remember these are ratios in molar amounts so you should keep them in balance. None of the reds I have created have these parameters. None the less I assumed the people who made this article knew way more than me so I spent THREE MONTHS! Trying to create a new matt glaze with the correct amount of alumina : silica while keeping the amounts of calcium, iron, phosphorus, and magnesium in line. From this we have this line test.
p.s At this point I start keeping notes in UMF molar amounts.
Sooner or later I reach a stopping point, keep in mind I’m only sowing a third of the tests here as I must have done about 30 firings. A this point I start making a Matt red glaze and playing with Mg0 amounts over 0.2 in the UMF. I’ll save you the trouble, they all turned out some variation of brown .
At this point I get frustrated and go back to what I know. Iv’e made plenty of oxidation iron saturated reds, Why am I throwing out all of my studies to someone elses? I throw one of my old reds together, up the Mg0, put the Ca0, iron, ect to the ratios I like them in and in the first shot I have success.
This is the final test so far. 0.13 molar amounts of Boron and 5% lithium. If I were to take a guess I think I can increase the boron further and take out even more lithium. Here's the last line test.
For anyone out following the same path as I am. I'm going to tell you what make a good iron saturated red regardless of these crystals. That article I linked was cheeks, doo doo even. I’ll even give it space to say I'm misinterpreting it. From my view it was wrong.
The chemistry You actually need for a good kaki oxidation red is (molar amounts)
Calcium (Ca0) between .35 - .4 Molar amount in the UMF . Too much will turn it bown
Phosphorus (P205) can be anywhere from 0.08 - 0.11
Magnesium (Mg0) can be between 0.3 - 0.35 Will it make your glaze a little more orange when higher amounts? yes but that's kind of what iron reds are in ox tbh.
Iron (Fe203) has to be high and pure. I find success between 0.15 - 0.19 molar amounts of iron.
Boron. Honestly the boron is up to you. I have recipes with no boron that make a very good red and this recipe here had a good amount of boron in it as well. It's all amount how much flux you want. Notice in the post I'm balancing the lithium amounts by increasing the boron? Just make it melt.
Final notes. In all reality good iron saturated oxidation reds are like low end crystal glazes with a balance of calcium, magnesium, iron, and phosphorus. That's all the are. Try to leave zinc out of the equation. I do not know why but zinc and iron do not get along too well. I’ll fully admit I do not know if the crystals I'm getting are coming from the calcium, magnesium, or iron, I don't really care after all of this to be honest with you. But if you are trying to make a good oxidation red, follow those parameters and you'll be fine. Almost all of my oxidation reds are with in those bounds and my reds seem far more red then theirs. Shout out to the John britt book about mid fire glazes. The book essentially told me its ok to have Mg0 in a iron saturated glaze even if it turns out a little orange because most if not all Kaki glazes (Iron saturated glazes like this) are a little orange.
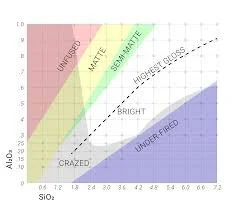
I find that most of these cone 6 oxidation saturated reds are right between where the stull chart turns into the archaic “craze” area and the normal bright area. One last thing. I've been testing the theory that this type of glaze is just a low end crystal glaze. Meaning, much like other crystal type glazes they are low in silica and alumina. I find that this is very true. If you have too much silica or alumina in the recipe it will make the chemical reactions the needs to take place for things to crystallize more difficult.
Good luck.